20+ Calculate The Equilibrium Constant For The Following Reaction At 25
In this experiment 288 g of gold is plated. Cus2Ag aqCu 2aq2Ags At 25 oC E cello 047 voltR8314 JK 1mol 1F96500 coulomb Easy.

Example 5 The Total Cost C X Associated With Production Of
Web Calculate the kinetic energy and the speed of the electrons ejected by light of wavelength i 650 nm ii 195 nm.

. CHEMISTRY The equilibrium constant Kc for the reaction I2 g. Web To determine the equilibrium constant for the above chemical reaction is Given the following data. Which of the following statements is or are true.
No MnO-_4 ions are. Temperature 25C The balanced chemical equation for the. Web 18 For a certain reaction A products a plot of lnA versus time produces a straight line with a slope of 3010 2 s 1.
Web And were given that the not for the reaction is -002 7 folds. So its important to remember that the equilibrium constant for a reaction is given by the following formula Where F. Web The equilibrium constant for the given reaction at 25C is.
Web Calculate the equilibrium constant for the following reaction. Sns Pb2 aq Sn2 aq Pbs The standard. See Answer Calculate the equilibrium constant for the following reaction at 25C.
Web Use the tabulated half-cell potentials to calculate the equilibrium constant mathrmK for the following balanced redox reaction at 25. Web Youll get a detailed solution from a subject matter expert that helps you learn core concepts. Calculate the equilibrium constant K for the following reaction at 25 C.
We know that 1mΔG ΔH -T ΔS and 2mΔG -RT lnK We can combine these equations to get 3m-RT lnK. Web Click hereto get an answer to your question Calculate the equilibrium constant for the following reaction at 25o C. Web The correct answer is C 14 104 Explanation.
How to calculate the equilibrium constant. Calculate the equilibrium constant of the following reaction at 25C from standard potential data. Web Search our solutions OR ask your own Custom question.
Calculate the equilibrium constant K for the following reaction at 25 C. Web An electrolysis experiment is performed to determine the value of the Faraday constant number of coulombs per mole of electrons. Web This overwhelmingly large equilibrium constant reflects the strength of permanganate ion as an oxidizing agent and that of zinc as a reducing agent.
It can be calculated by the Nernst equation at.

Solved 1 The Equilibrium Constant Is Glven For One Of The Chegg Com

Calculate The Potential For Half Cell Containing 0 10 M K2cr2 O7 Aq 0 20 M Cr 3 Aq And 1 0 10 4 M H Aq The Half Cell Reaction Is And

Droste Chapter 2 3 Pdf Electrochemistry Gibbs Free Energy

For The Reaction C2h4 G H2 G Rarr C2h6 G Kp 3 356 Xx 10 17 Calculate Deltag For The Reaction At 25 C

All Abstracts For The Icos Science Conference 2020 Icos

Speciation Of Yttrium And The Rare Earth Elements In Seawater Review Of A 20 Year Analytical Journey Sciencedirect

Equilibrium Constants For Pb And Cd At 25c Download Table
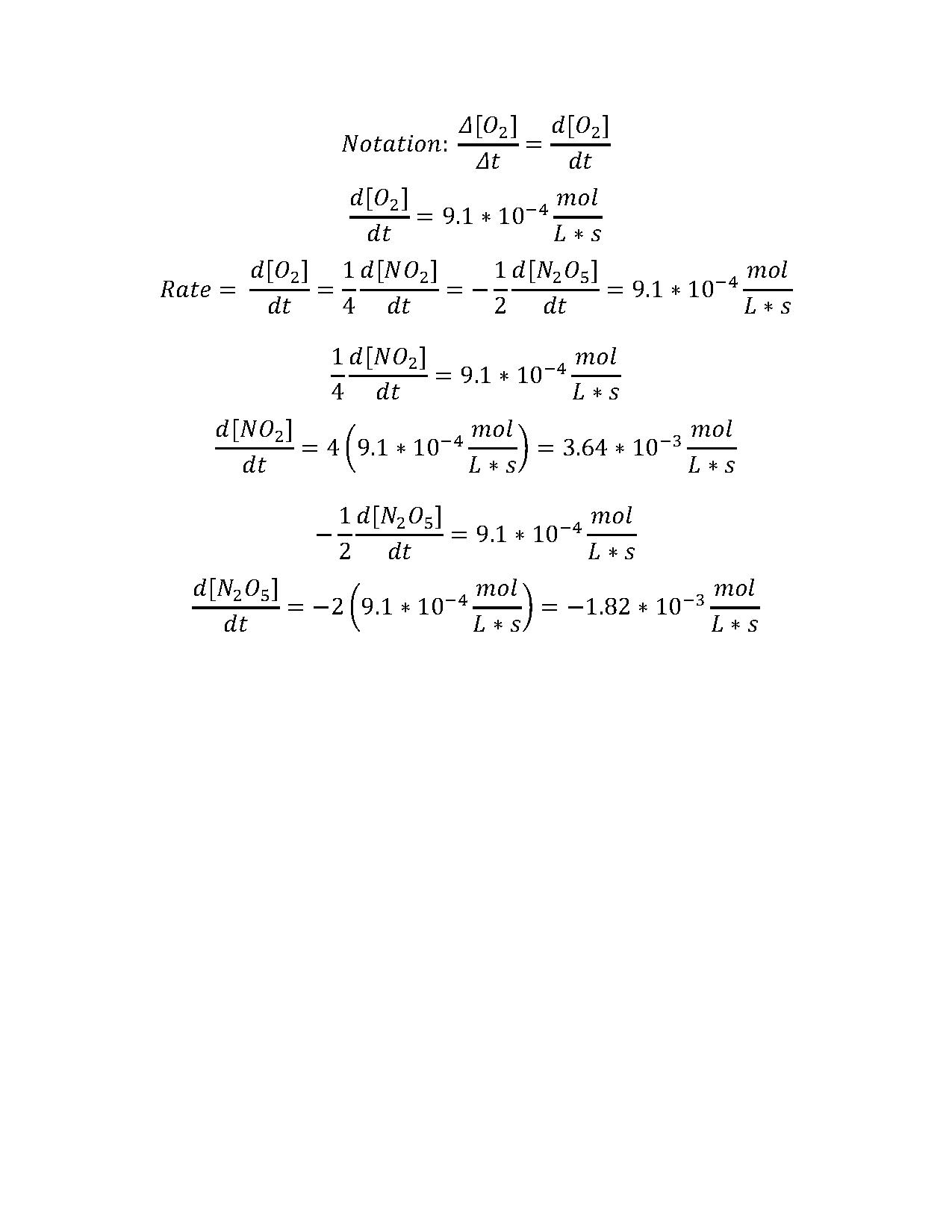
Hw Solutions 8 Chemistry Libretexts

Solved 17 Calculate The Equilibrium Constants For The Chegg Com
Frontiers Best Practices In Pec Water Splitting How To Reliably Measure Solar To Hydrogen Efficiency Of Photoelectrodes
21st Isop Annual Meeting A New Era Of Pharmacovigilance Challenges And Opportunities 20 23 September 2022 Verona Italy Springerlink

Interface Vol 30 No 4 Winter 2021 By The Electrochemical Society Issuu

Calculate The Equilibrium Constant For The Reaction At 25 Oc Cu S 2ag Aq Cu 2 Aq 2ag S At 25 Oc E O Cell 0 47 V R 8 134 Jk 1 F 96500 C Is
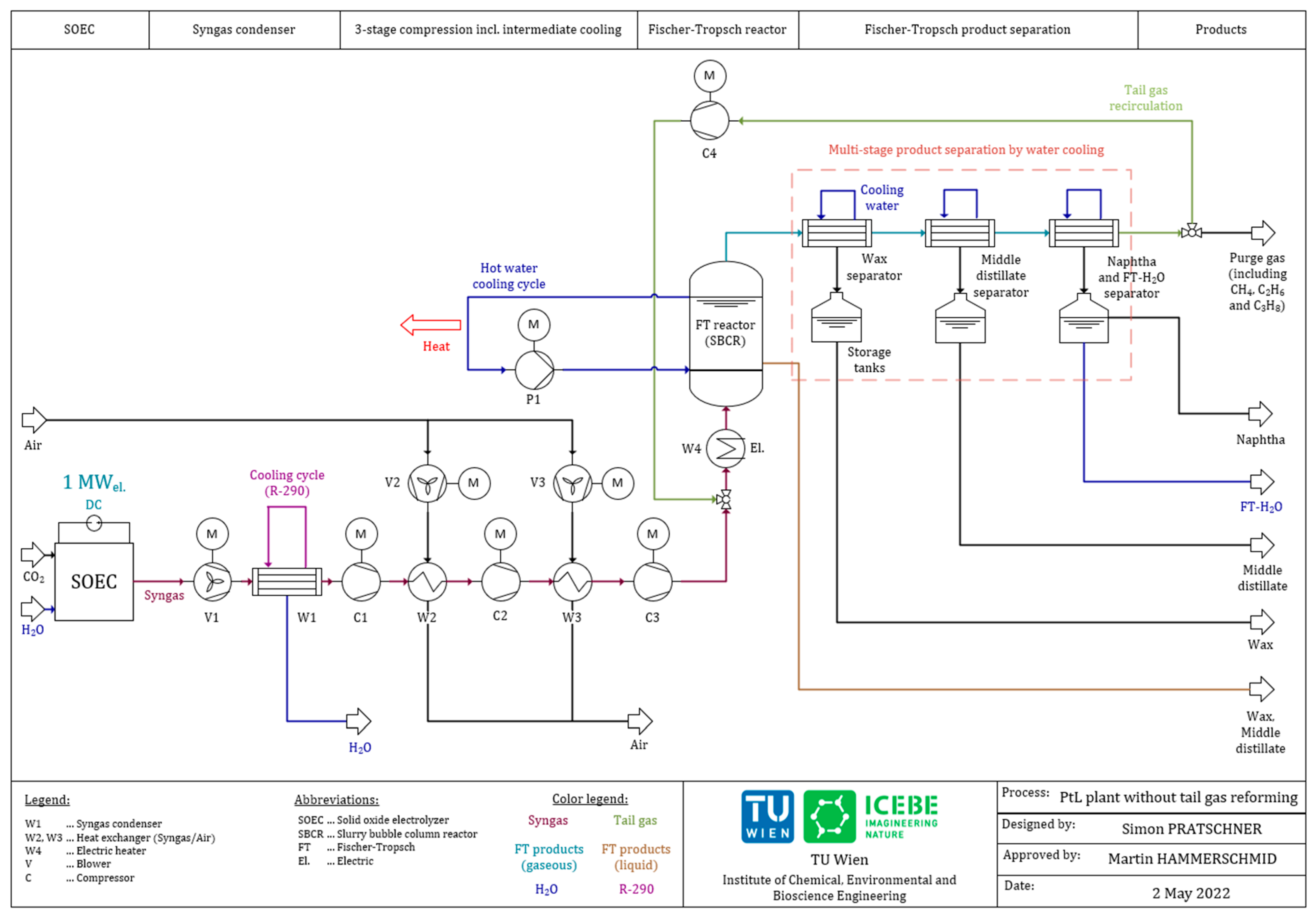
Energies Free Full Text Simulation Of A Pilot Scale Power To Liquid Plant Producing Synthetic Fuel And Wax By Combining Fischer Ndash Tropsch Synthesis And Soec Html

The Equilibrium Constant For The Following Reaction At 25 O C Is 2 9 10 9 Calculate Standard Voltage Of The Cell Cl2 G Br Aq 2cl Aq
What Is The Equivalent Weight Of Fes For The Reaction Fes Fe3 So3 Quora

The Following Reaction Is Performed At 298 K 2no G O2 G 2no2 G The Standard Free Energy Of Formation Of No G Is 86 6 Kj Mol At 298 K What Is The Standard Free Energy